 News
Articles & Recipes
Articles
The Ground Beneath Us
News
Articles & Recipes
Articles
The Ground Beneath Us
The Ground Beneath Us
Not a day goes by when someone is shouting about lead in their lipstick, asking if this material is 'free from nanoparticles' questioning the levels of asbestos in talc or arsenic in rice. While these are all good and legitimate questions it does seem that we have lost touch with the origin of all things natural, the soil.
Natural cosmetics demand natural ingredients and that usually means ingredients that have grown in or around the soil. Those of you who look out for or manufacture organic products know that human activity can damage the gentle balance within the soil rendering it quite toxic and totally unsuitable for farming and that is one appeal of the standard - no pesticides, no herbicides, no fertilizers, no 'nasties'. But is that true? Further, is that all we have to look out for? We decided to take a closer look at soil in its natural state to see what lies in the ground beneath us.
What is soil?
Just like deep-sea oil, soil takes many thousands of years to form, slowly building over time from the weathering of rock and the decay of animal and plant material. Soil quality rises in line with plant and animal life as each one serves to nourish and protect the other, plant roots prevent soil erosion, plant life supports animal life, animal droppings feed nutrients back to soil and eventually everything returns to soil ready for it all to begin again and proving that there is indeed life after death.
Is all soil created equally?
With soil being such a complex mixture of animal, vegetable and mineral it isn't too difficult to imagine how and why soil differs from one location to another. It could be argued that gardening is as much chemistry as hard physical labour and imagination - something that has long been appreciated by factory farmers and the big seed companies.
The rock that feeds into the structure and body of the soil starts the ball rolling and it is here that we must also look for answers to our cosmetic 'free from' claims.
Hard Rock vs. Heavy Metal :
If you have ever taken the time to look at a specification sheet for your herbal extracts and botanicals you would have found something like this:
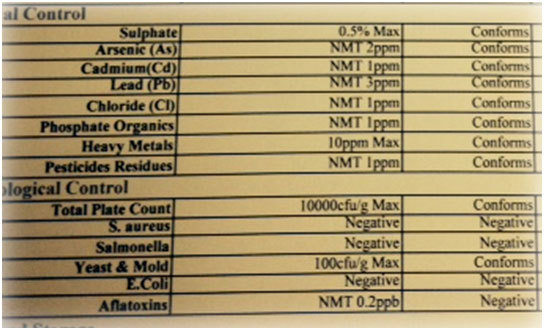
While it is great to have analytical data to hand it means nothing without the ability to both interpret it and to apply it to your own personal situation and that is what we will look at next.
Rocks contain metals and a quick look at the periodic table will remind us that there are quite a few metals that make up the earth including some that are toxic, the heavy metals. It is not uncommon to find traces of these heavy metals in plant material and yes this includes lead!
One common phrase used by fear mongering groups is that 'there is no safe level this or that heavy metal' and that we should eradicate it completely from our skin diet (and especially from our food I guess). I can see their point and yes, heavy metals are toxic and dangerous for health, there is no doubt about that but can we really banish them completely? Especially when trace heavy metals are as natural as any other mineral or vegetable that can be found if we look close enough. However, it is very important to look at the human influence (industry, chemicals, farming practices etc.) vs. what nature provides as surely if we can make a safer choice (possibly organic farming?) then wouldn't it make sense to do so...
Analysing the data:
Our first step towards understanding the above is getting a grip with numbers and I know how much you all love that!
PPM = Parts Per Million.
The ratio of one thing against another like one green ball in a total of 1 million balls.
or
One second in 11.5 days
One minute in two years
Chemists use Parts Per Million when talking about solutions. This ratio is also used when communicating information about pollutants or trace chemicals in the environment. We might say that the river contained 5 parts of diesel oil in 1 million parts river.While this gives us some idea that the pollution is only relatively small we need more than just the numbers (or ratio) to help us decide if that is a problem or not.
Take something as simple as salt (Sodium Chloride) for example.
Fresh water is often defined as water which has less than 1000 parts per million of dissolved salts.
Brackish water can have anywhere from 1000-35,000 ppm of salt per million parts water.
Seawater has between 30,000-50,000 ppm salt per million water.
The dead sea has around 330,000 parts salt per million parts sea water meaning that it is 1/3rd salt!
If we wanted to evaluate water for drinking then anything over the fresh water level of salt would be a problem. Sea Water is unsafe for drinking straight from the sea.
If we were talking about water fit for swimming in then we might conclude that all of the above is OK for swimming based on the information we have.
If we are trying to work out if the water is safe for fish then we can't answer that question until we know something more about the fish - we can all think of fish that thrive in the sea vs. fish that prefer fresh water.
As humans our ability to thrive, tolerate or be destroyed by these 'parts per million' materials is dependent on many things and not just the fact that there is a 'part per million' impurity present. It helps to know where these materials came from, what form they take in the environment and how that might affect us.
Impurities in Soil - Where from?
The natural background concentration of heavy metals in soil can be defined as the concentration that exists outside of human activities.All elements known to man (the periodic table) are 'natural' and exist somewhere on this planet so it makes sense that sometimes we find things in our soil, air or water that don't thrill us and fill us with excitement.
Establishing what is 'natural' in terms of heavy metals is difficult as it is impossible to find samples of soil that existed before human intervention but analysis carried out by many countries and comparisons between farmed, industrial and virgin landfall has given us these rough guidelines:
| Arsenic | 0.8-3.7 ppm |
| Cadmium | 0.5 - 1.5 ppm |
| Chromium | 40-100 ppm |
| Copper | 35-50 ppm |
| Lead | 17-85 ppm |
| Zinc | 60-140 ppm |
(Amalgamation of data from Canada, Netherlands and Brazil).
The reason that these will always be ranges rather than absolute figures is due to the variation of soil types around the world. Some soils will always contain more heavy metal impurities than others).
So, now that we have some idea of what exists in natural, un-touched, virgin soil we can have a look at two of the key 'cosmetic' impurities in a bid to work out how they might affect our health and wellbeing.
Arsenic
1.8 ppm of Arsenic exists naturally in the Earths crust.
You might have been surprised to notice that the above cosmetic grade material has a specification that allows for up to 2ppm Arsenic, especially knowing that Arsenic is pretty nasty stuff but as we have seen above it is also very natural.
Arsenic is often associated with sulfur deposits commonly found in and around volcanic soil.In terms of human health it is a known carcinogen and is made more dangerous by the fact that it can enter the body through ingestion, inhalation or skin absorption (although this is by far the least likely route) depending on its form. Once in the body it can affect the lungs, liver, kidneys and skin.
While Arsenic is definitely not something to seek out in ones diet or skin care the body can process trace doses of arsenic by excreting them in the usual manor. Whether or not it does this depends on the format the arsenic is delivered in.
Inorganic vs. Organic Arsenic.
Arsenic exists in the environment in association with other chemical elements and we have tended to refer to these mixtures (or compounds) as Inorganic or Organic arsenic. These are both chemical rather than marketing terms and refer to whether the arsenic is bound in a mixture containing carbon (organic) or not.Contrary to popular belief both types of arsenic mixture occur naturally and both have industrial applications. Practically speaking this means that it is near on impossible to completely avoid arsenic.
Inorganic Arsenic is often associated with wood preservatives - the most common source of environmental contamination for most people. Used as preservatives, termite barriers and insecticides arsenic treated wood has a long history of use for outdoor decks, playgrounds and railway sleepers. It is also commonly found around mines and industrial parks where it is used in metal smelting.
Inorganic arsenic has had the largest negative effect on human health through contaminated drinking or ground water and soil. It is less of a problem in food but can cause issues in rice and carrots due to the nature of how these plants take up minerals.
Organic Arsenic is often found in pesticides used in agriculture and can enter the food chain via the plants or soil. Organic arsenic can often be found in fish including shellfish and in some vegetable crops.
The biology of Arsenic metabolism is a little too complex to cover in this article but of the two types Inorganic compounds are the most likely to accumulate in the body and cause poisoning (although it shouldn't be assumed that all inorganic arsenic WILL accumulate, our bodies are good at excreting what doesn't serve us given time) although all types of arsenic exposure should be limited. Food standards agencies, environmental protection agencies and the cosmetics industry set safety guidelines, which under normal circumstances should not cause long-term health issues.
For the cosmetic chemist the take-home message is that there will be some Arsenic in many natural extracts and materials but levels in the raw materials are usually low and manageable without detrimental effect.
Lead:
Naturally occurring lead accounts for about 14 ppm of the Earths Crust. Compare that to Oxygen, which sits at around 461,000 ppm, Iron at 56,300 ppm, Arsenic at 1.8 ppm, Gold 0.004 ppm and Silver 0.075 ppm.
While it is true that Lead is naturally occurring and also that all of the lead found on this planet has been here all along it isn't right to say that it is just natural and we shouldn't worry about it.
Lead is a relatively easy metal to mine and is often found close to the surface bound in ore with Silver, Zinc and Copper. Research into lead concentrations in soil across Europe have been affected by anthropomorphic (human) activity since approx. 5000 BC with massive increases in Soil bound lead relating to the Greek-Roman period of around 0 AD, another peak at 1000 AD as metal technology took another leap forward then a further rise in 1530AD when metallurgy reached a peak and then onto the Industrial Revolution, World War two then the post war boom until reaching a peak in the early 1970's.
Legislation to reduce lead in paint and remove lead from fuel has seen lead levels in the environment decrease rapidly since the 1970's but the lead that we measure today both in our soil and water is mostly a hang over from these days of reckless abandon.
The reason that this anthropomorphic lead pollution is such a problem is because once released from its natural ore it tends to sit in the soil or water without degrading. It can also bio-accumulate in animals and make its way up the food chain. It also leaches into vegetation hence many of our cosmetic botanicals having a specification for lead. Sadly as much of the lead pollution we find spread far and wide originated from vehicle exhausts and was taken up into the atmosphere before falling as rain or settling as dust it is impossible to completely avoid it and this is one reason why campaigns for 'getting the lead out of my lipstick or whatever' serve more to spread fear than change protocols. Make your lipsticks or other cosmetics from organic botanicals grown in a Swedish virgin forest and it is likely that lead would still be on the menu.
As colour cosmetics remain the cosmetic type most likely to increase your daily dose of the Lead stuff we wanted to find out why?
Iron Oxides are common in colour cosmetics and especially in foundations (including mineral powders), blushers, eye shadows and some lipsticks. Iron Oxides (natural minerals) have been shown to help remove lead from the environment by forming a connection with it. While this is a great thing for Mother Nature it may not be so neat for you or your mother who then goes on to apply said lead-enriched colourant onto their face.
That said the story is scarier than reality in all but the very worst of cases as cosmetic regulations around the world measure for lead levels in pigments and restrict the amount that can be present in a colour product.Synthetically produced Iron Oxide colourants contain far less lead (typical background levels really) than their natural counterparts.
Other cosmetic colourants built on Chromium, barium, strontium and zirconium lakes (FD&C colourants) and natural colourants such as Beta Carotene also come under these same guidelines. The reason that these non iron oxide pigments contain lead is because of the metal salts used in their processing. The metals have all at one time or another been extracted from natural Ore and those ores contain varying amounts of Lead depending on their chemistry, location and concentration. There is just no getting away from it!
So what use is all of this to the Cosmetic formulator?
Cosmetics are meant to make us feel and look good, in the best of cases they should help to make life more beautiful, brighter, and better even.They should never, ever make us sick. Regulations exist to help the cosmetics formulator make products that fulfill these outcomes to the best of our knowledge and abilities but the question is, is this regulation good enough?
Today many of us have access to more information than we have time to comprehend or fully digest it. We discover that there is Lead in our lipstick and feel conned, cheated, mis-lead. However, how many of us would stop to ask about background lead levels (at an average of 14 parts per million occurring naturally our cosmetic ingredient with a specification of less than 3 ppm is looking out-of-this-world good!
We know that Arsenic is a deadly poison much loved by murder mystery writers but didn't appreciate that it could also be found in our health foods (Brown rice, carrots and certain types of seaweed are renown for having higher than average levels of arsenic present due to the way they grow). Again we could begin to think that we are better off without our daily wash and make-up but again with a spec of less than 2 ppm compared to a natural concentration of roughly 1.8ppm would the net result of abstaining from cosmetic consumption really impact our Arsenic dose?
While it isn't helpful to dismiss Lead, Arsenic or any other heavy-metal pollution out of hand - yes it is natural but it didn't get put on the earth in this easy-to-consume-state WE MADE THAT - it is also not entirely useful to set ourselves up for a life so clean that it we need to vacate this earth to live it.
From where I am sitting it seems like a little bit of natural skin care and some make-up every now and then probably won't kill you whereas on the other hand, worrying about it all might.
Oh and before I forget what about nanoparticles? Well I blame volcanoes and bush fires for the inspiration - Nature's own Nano-spray guns.
Amanda Foxon-Hill
29 April 2014
