 News
Articles & Recipes
Articles
An Introduction to pH, pH Adjusting and Formula...
News
Articles & Recipes
Articles
An Introduction to pH, pH Adjusting and Formula...
An Introduction to pH, pH Adjusting and Formulating with Cosmetic Acids
Hydrogen ions, what are they and where do they come from?
Hydrogen is a chemical element that exists all over this planet. It is found in water, in your body, in fruits and vegetables, the soil and also in space.
Hydrogen is the lightest of the chemical elements and it has in its nature, an inherent neediness that motivates it to seek out relationships with other chemicals in order to bring it into balance.
If hydrogen could exist in its own universe with no other chemicals around it would exist most comfortably in a H-H pair instead of bobbing along forever on its own or being in an un-equal relationship with something else.
As we don't live in a hydrogen-only world, a fair proportion of the hydrogen we encounter in a cosmetic formula is in a relationship with carbon, oxygen, sulphur or nitrogen and mostly it stays bonded that way.
Hydrogen ions are formed when the relationship that hydrogen is in breaks down and the independent hydrogen is set free. Hydrogen typically comes away from that scenario with a single positive charge and that's what pH meters measure.
Free vs Bonded Hydrogen
While most hydrogen in your formula will remain bonded to its parent material throughout your products shelf life some may break free and form ions over time during oxidation reactions. While these releases can be damaging to the product and change its pH, these pH changes are usually quite small and often the changes in a products colour, odour or form are more noticeable and problematic.
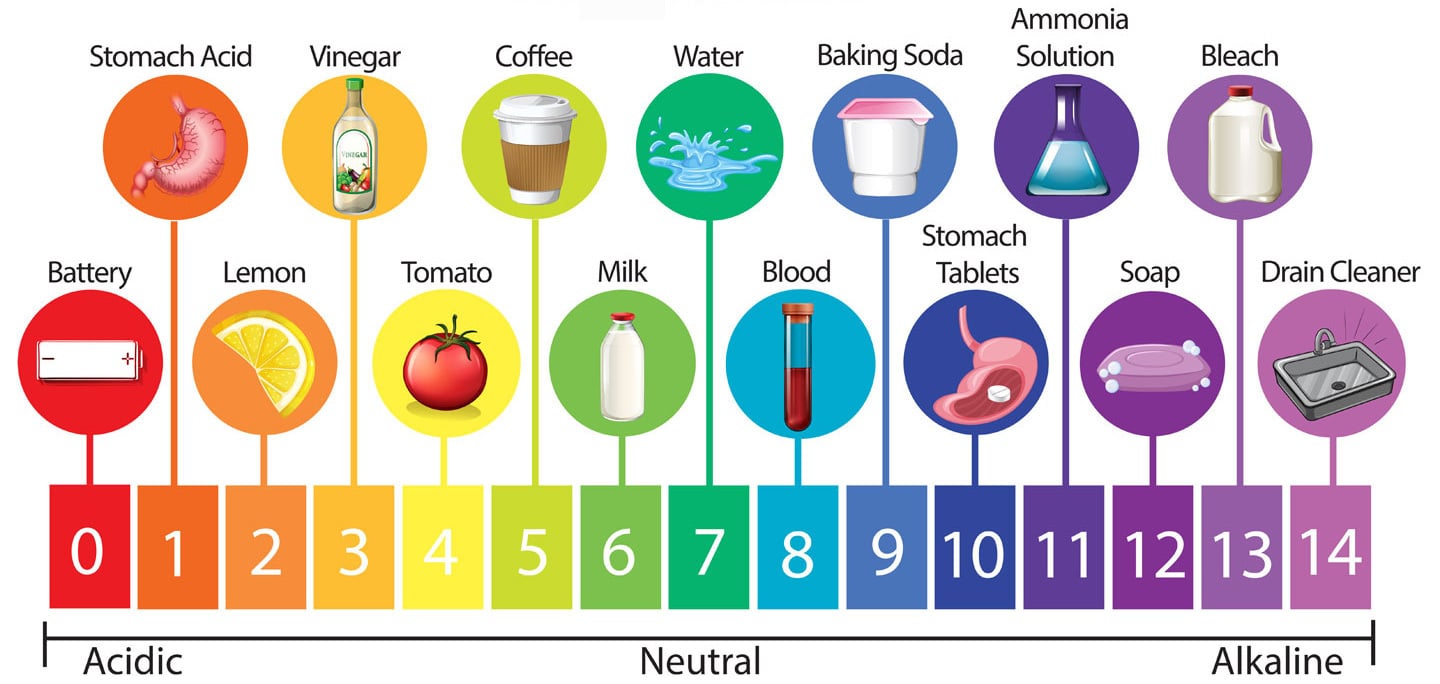
Formulating Chemistry - What is a good pH to aim for?
There is no singular answer to this question as it depends heavily on your product ingredient philosophy, your formula chemistry and what action you want to achieve from your product.
In skin terms, the cosmetic industry generally regards pH 5.5 as skin-kind or balanced. That doesn't mean a product with a pH slightly or even quite a bit higher or lower is no good or will be felt as harsh in any way. It's more that pH 5.5 is an average measure for 'normal' skin. This pH, being slightly acidic and about half way between the maximum and minimum values typically recorded on adult skin is the pH that we can expect healthy skin to try and return to given time. It reflects the natural acidity of our microbiome and skin secretions and the point at which our skin is most likely to be relaxed.
Understanding pH is central to understanding the skin and cosmetic science. As such, cosmetic formulators cannot make safe, stable and effective cosmetics without understanding a little about pH and how it relates to their product both as it is used and as it exists as a formula.
Do all products have a pH?
No, oil-only formulations do not have a pH.
Waterless (anhydrous) or water-light (low levels or tightly bound/ more static water phase) products such as oil-only balms, salt scrubs, powdered clay masks , bars of soap and solid deodorant sticks don't typically have a measurable pH as supplied whereas most emulsions, suspensions and solubilised spritz-type formulations do.
Thinking 'but soap bars have a pH, we talk about it all the time?'
Solid (or dry) products that soften, blend or extend into water can and often do return a pH reading but only when wet rather than dry. In the dry state the hydrogens are still largely bonded to their parents rather than wandering around free where they can be measured! pH strips or probes need to be wet before they can detect hydrogen ions and we often achieve that by placing a little of the dry good into demineralised water (pH 7) prior to measuring it.
Deodorant sticks that contain bicarbonate of soda are another example of a dry product that will record a pH once wet. With soap the pH becomes apparent as we use the product with water to wash our skin. With a deodorant the pH of the product develops as the product mixes with sweat from the surface of the skin. While a bicarb-based deodorant stick is not necessarily applied to wet skin, certainly not in the way that soap is, it will become wet during use (from sweat). For this reason, it is a great idea to test these products when dampened enough to elicit a pH measurement as this helps the formulator understand how the skin will experience it during use.
How does the skin react to pH?
As we have seen already, our skin is naturally acidic resting anywhere from pH 4.5 through to 6.5. Neutral, or the balance point of the pH scale is 7, the pH of pure demineralised water. It is best to think of our skin as existing in an environment that has a range rather than set pH - a dynamic rather than static situation that changes in response to its hydration status, our individual chemical soup, the products we've recently (or are) using, our age and hormones plus a few other factors. Skin can cope with being exposed to pH's outside of its resting state over both the short and long term just as long as the product is not too chemically or physiologically harsh. The human body is used to existing in an ever-changing world and the skin it's no different, it will work to restore balance just as long as it has the time, space and resources to do so.
Can we feel pH?
In many cases, healthy skin starts to 'feel' pH when it is either very low (pH 1-3.5) or high (pH 9.5-14) and when the skin has had enough time to penetrate past our corneocyte layer. It is reasonable to expect damaged, inflamed or thinner skin to respond sooner and/or more dramatically to pH levels less extreme than this but for the most part, our sensory experience of pH as a stand-alone measure of a formula's skin-compatibility is less dramatic than we may have first thought.
Why does pH matter in a formula?
Measuring and then managing pH enables us to step in and make any adjustments necessary for protecting the integrity and stability of a particular ingredient. It may also be necessary to have a particular pH to increase the likelihood of your formula working, especially when a particular active is more soluble, active or bioavailable at a particular pH. In another scenario we may need to formulate to a certain pH for our chosen preservative strategy to work. These days many people formulate to a natural strategy, using organic acids as part of their antimicrobial strategy. The majority of these require a pH of 6 or under for them to be active and available in the right spot in the formula, creating a formula that sits outside of this may lead to microbial failure or a crystallising out of the preservative.
There are also cases where we wish to call our product 'pH balanced' or 'skin kind' and adjusting the formula pH helps us achieve that.
In some select scenarios adjusting pH increases or decreases the viscosity of our product, either by neutralising a thickener (as is the case with many carbomer polymers) or by changing the chemistry or physical orientation of another ingredient, such as a clay or other ionic active. One additional aspect that can be affected by pH change is colour with many natural pigments and dyes shifting their shade or colour intensity in response to pH.
Do all formulations with a pH need it adjusting?
No, it is not always necessary to adjust a formula pH at the end of manufacturing or sample preparation. It is, however, important to measure, record and reflect on what that pH means for your product's microbial and physical stability, its functionality and how the skin may respond to it.
I didn't add any acids or alkalis so what made my formula pH change?
Remember that pH is a measure of hydrogen ions in water. Pure, demineralised water has a pH of 7. Acidic ingredients will increase the number of hydrogen ions in solution, basic ingredients will reduce them. The pH you end up with will reflect the overall change in your formula status.
When we are formulating and have reached the final step of checking the product pH we may find it to be neutral (pH 7), acidic (pH 6.9 or less) or alkali (pH 7.1 or more). As pH can be altered by any ingredient that releases or collects hydrogen ions in your formula's water phase, it's quite possible that your final formula has a pH that has shifted away from neutral even when you haven't added anything obviously acidic or alkali along the way.
Ingredients commonly added to every-day formulations that can alter formula pH quite dramatically without us wanting them to include chelating agents (EDTA, Sodium Phytate) and some Surfactants (Castile Soap, Glucosides) which tend to shift pH up and Fruit juice powders, vitamin C, dead sea mud, kaolin and natural preservatives that often shift pH down. These ingredients alter pH because they contain some acid or alkali chemistry but we may not think of them that way when we are doing our formulating.
Another scenario exists where ingredients we expect to alter formula pH in a predictable way don't. Hyaluronic Acid is a good example of an ingredient we assume is acid but has little effect on the formula pH. This is because it is typically sold in its sodium salt form and this has an as-supplied pH closer to 7. Other surprising acids exist such as the Amino Acid Glycine which has a resting pH of 6 and Arginine which is another which has a natural pH of 12.6!
The take-home message is if you are formulating a multi-ingredient product with a water phase that can host hydrogen ion exchanges assume nothing and make sure you measure your pH!
Adjusting your formula pH
| Potential pH Adjuster | pH Change from pH 7 | Material Origin | Benefits | Risks |
| Citric Acid | Reduce | Natural, fermentation of corn | Cheap, natural, good water solubility, colourless & odourless, skin compatible and gentle salts (hydrating). | Some people feel they are sensitive to citric but there is limited evidence to support that. |
| Lactic Acid | Reduce | Natural, microbial fermentation | Natural, good water solubility, doubles up as skin active | More expensive, higher level of skin activity than citric, slight odour |
| L-Arginine or Lysine HCL | Increase | Predominantly via microbial Fermentation | Skin-compatible amino-acid, Collagen booster | Expensive compared to other adjusters. Undergoes a wide range of chemical reactions, many of which are unhelpful and unwanted in a natural formula, odour and light colour, limited water solubility, slow to change pH |
| Triethanolamine | Increase | Synthetic Amine chemistry | Fairly cost-effective, can form more than one bond so can increase the strength of carbomer gels. | Amines can create bi-products in a formula, slight smell, slightly increases potential for skin irritation, light colour. More costly and reactive than alternatives |
| Sodium or Potassium Hydroxide | Increase | Electrolysis of salt (NaCl) or Electrolysis of Potassium Chloride which is extracted from sea water or minerals | Very cheap, neutralises to salt and water only so very safe, highly effective at changing pH, little impact on product stability under normal use | Seen as synthetic chemical (more accurate to call it an industrial chemical vs synthetic). Seen as harsh. Not allowed in organics other than for soap making. |
| Aminomethyl Propanol | Increase | Synthetic Alkanolamide made from amino acid | Speciality additive for certain cosmetic ingredients. | Amine chemistry can cause issues in formulations when not managed |
| Sodium Bicarbonate | Increase | Mined or produced from sodium carbonate | Skin-softening, cleansing/ soap like action, gentle and natural, relatively low priced | Very slow to change pH, Neutralisation reactions can salt-out a formula, poor water solubility, can leave product with gritty look and feel. Generated gas during neutralisation and this can aerate a product (CO2) |
Choosing ingredients for pH modification
Where it is necessary or desirable to change the formula pH we typically use citric acid to adjust the pH down (to become more acidic) and Sodium Hydroxide to bring the pH up (more alkali).
Citric Acid and Sodium Hydroxide have a number of features that make them attractive for use in this context including:
- Highly efficient
- Cost effective
- Widely available
- Available in pure form
- Undergo highly predictable and relatively simple neutralisation reactions with skin-friendly, non-problematic bi-products (salts and water)
- Readily water soluble
- Odourless and colourless in solution
The most common way to use these chemicals is to first create 20 - 30% solutions with demineralised water and then add them little by little into the formula using the pH reading to guide you when the end point has been reached. In formulating terminology, we note the amount needed as 'q.s' meaning 'quantity sufficient'. Sufficient for what you might ask to which the answer is to achieve the pH you state for the formula. pH adjustment steps rarely require more than a few drops of either of the above to shift the pH to where you want it, especially when these pH adjusters are used. Alternative pH adjusting chemicals such as Triethanolamine (to raise pH) and lactic acid (to lower pH) can be useful in some formulation settings but be warned, not all acidic or alkali chemicals are well suited to cosmetic pH adjustment and some will create more problems than they solve! Bicarbonate of soda and amino acids for increasing pH are good examples of where pH adjustment can easily go wrong, creating formula instability and adding unnecessary costs and chaos!
Formulations that feature pH as part of their functionality
Formulations that feature AHA's as key actives are where we see pH get the most attention in cosmetic science.
AHA's are a particular family of acids that are known for their skin regenerative and correctional properties. They can boost collagen production, smooth the skin, increase cell turn-over, brighten the complexion and increase cellular hydration when formulated appropriately. The two acids that receive the most attention are Lactic and Glycolic both of which are best formulated to pH of 4 or less but as we will now see, pH isn't everything.
What pH can and can't tell us
As we found out earlier, pH is measuring the number of hydrogen ions in solution and one of the most popular ways we end up with lots of those on purpose is in an AHA formula.
While pH does reflect the concentration of the acid present it does so via a logarithmic rather than step-by-step scale. This means you could have two cosmetic products presenting with roughly the same pH but that contain very different percentage inputs of acid. While the chemists out there will know that you absolutely can work out the amount of acid present in a solution if you know both what you are looking for and the solution pH, cosmetic formulations are rarely that simple. We must keep in mind that the product pH reflects ALL the hydrogen in the formula and they could have come from quite a few different ingredients.
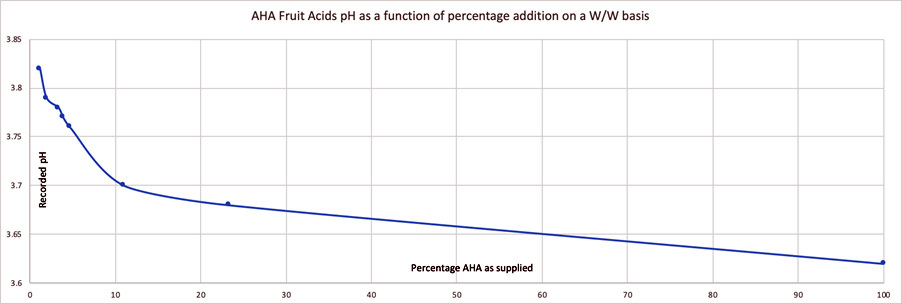
Acknowledging the formula pH alone doesn't provide enough information for one to work out just how strong the product will feel and what it might be able to achieve on the skin is easier understood with an example. I took an ingredient called 'AHA Fruit Acids' and measured its pH in solutions of decreasing activity, starting with the acid blend as supplied and moving to it being just 1% active (as supplied) in the solution. The graph below shows a pH change of only 0.1 units between the 1% and 10% concentration samples. That same is true for the 10% vs 100% acid samples. Very small pH changes in progressive dilutions are typical for the acids we use in cosmetic science:
Introducing pKa
In spite of the fact that an acid pH is anything below pH 7, we tend to formulate AHA products at a pH of 4 or less because that's the pH range at which they are most likely to penetrate the skin and act.
An acids pKa values gives us some very specific and useful information that is independent of its concentration. The pKa is the pH value at which the acid exists in an equilibrium of 50% acid form and 50% salt plus hydrogen ion form. Increasing the pH beyond the pKa pushes the equilibrium to the right meaning the acid exists mainly in its salt form. Reducing the pH pushes the equilibrium to the left meaning it exists mainly in its acid form in solution.
A useful analogy for the impact of formulating at or below the pKa is achieved by using a vehicle. The vehicle type is the acid, the pH if the amount of fuel in the engine, the distance from the pKa is the amount the driver pushes down the accelerator.
Compare two AHA formulations, both which contain 10% Lactic Acid and all other formula components being equal except for one thing. Formula A has its pH adjusted to 3 while formula B has a pH 4. In formula A the lower pH acts like an accelerator pressed to the floor meaning the product zooms deeper into the skin. In formula B the journey is undertaken more slowly. Given enough contact time the two products could, theoretically achieve the same results but whether they do or not depends on how the product is designed to be used (leave on vs rinse off and if rinse off is the contact fleeting or extended?) and the speed in which the skin responds (and counters) their attack! As people always ask 'but which is best' I will pro-actively answer by stating if this hypothesis is true, it wouldn't be a case of there being a single best, more that there would be a best fit for the scenario you had in mind! Another applied solution!
To summarise, cosmetic chemists take into consideration both pKa and formula pH when creating AHA products to help them dial the products activity up or down.
How much acid is enough?
If we go back to our vehicle analogy, the amount of acid added (the percentage) could be thought of as the amount of fuel in the tank. The more fuel, the further you can go remembering the acid pKa plus formula pH tell you how fast they will get there. AHA's such as Glycolic, Lactic and popular AHA Fruit Acid blends have no minimum level to use as any amount of acid will do something, either to the skin or to the formula pH. In terms of maximum, cosmetic safety guidelines strongly recommend 30% as the upper limit, especially for the stronger Glycolic and Lactic Acids which can switch from being anti-ageing and helpful to damaging when they are used recklessly. In all cases, sun protection is a must for people using acidic skincare as part of a regular regimen.
Common AHA formulations contain one or more acids formulated with between 5-30% acid on a per actives basis with the higher acid containing products mainly focusing on lactic and/or glycolic acid. Acetic acid which is also known as vinegar is not often thought of as dangerous but is, in fact corrosive when used on the skin in concentrations of 25% or more. While relatively strong, both Lactic and Glycolic are safer than acetic at these higher levels.
When calculating percentage input keep in mind most AHA's are supplied as diluted liquids with Glycolic mostly supplied as a 70% solution and Lactic an 80% solution. Household vinegar including apple cider vinegar is usually between 4-8% active.
What difference does the acid type make?
We can rank the potential (or maximum performance) of a cosmetic acid by reviewing its molecular weight, acid type (chemistry) pKa and water solubility.
| Acid | pKa | Molecular Weight g/mol | Acid Type | Originally Discovered in | Solubility in water at room temp (21°C) |
| Acetic | 4.756 | 60.052 | Carboxylic AHA | Vinegar | Water Miscible |
| Citric | 3.13, 4.76, 6.4 | 192.123 | Carboxylic AHA | Citrus Fruits | 59% at 20C |
| Hyaluronic | NA | 403.31 (monomer) | Glycosaminoglycan | Human Body, Rooster Combs | Swellable |
| Gluconic Acid | 3.86 | 196.155 | Carboxylic | Honey | 31% |
| Lactic | 3.86, 15.1 | 90.078 | AHA | Milk, Muscles | Miscible |
| Lactobionic (Patent protected in cosmetics) | 3.8 | 358.296 | Disaccharide (PHA) | Sugars | Freely Soluble |
| Glycolic | 3.83 | 76.05 | AHA | Sugar Cane | To 70% at room temp |
| Malic | 3.2 | 134.09 | Dicarboxylic | Apple | 55% in water at room temp |
| Mandelic | 3.41 | 152.149 | Aromatic AHA | Almond | 15.8% in water at room temp |
| Tartaric Acid | 2.89, 4.4 | 150.087 | Carboxylic AHA | Grapes, Bananas, Tamarinds | Highly Soluble |
| Salicylic | 2.97, 13.82 | 138.122 | BHA | Willow | 0.25% at room temp in water |
| Amino Acids | Various | Various | Amino Acids | Human body, Food Sources | Various |
Sticking with our car analogy we can imagine our individual acids as types of vehicle and visualise their performance that way. Cosmetic acids could be classified as either formula 1 sports cars, hot hatches or high-spec family wagons based on a review of the criteria above. Using that analogy Acetic Acid would be your formula 1 car it being extremely powerful, somewhat impractical in most cases and definitely with the potential to be dangerous.
Next up are Glycolic and Lactic with Glycolic the most potent of the two. These are your hot hatches - versatile, fairly controllable but given the chance, very high performing and a lot easier to control than your formula one friend!
High spec family wagons come next and, in that class, we have Citric, Malic, Mandelic, Gluconic and Salicylic. These are characterised not only by their larger molecular weights (bigger size, slower action) but also by the comfort of their action being most able to give achieve results without the irritation and discomfort.
Malic and Mandelic have the potential to give stronger results but are limited by their relatively low water solubility and /or very low pKa meaning in all over-the-counter formulations their salt form will massively predominate.
Salicylic is in this category as while it's pKa is very low indicating its potentially stronger than glycolic, it is not very water soluble in that form and so is mostly formulated into oils or emulsions which slow down its passage somewhat (but also make it more comfortable to use) or it is kept in its more water-soluble salt form and added that way.
Gluconic or honey acid is possibly the most multi-functional of the options switching to its salt form, acting as a chelating agent and functioning as a microbiome boosting sugar as well as having acidic functionality. For these reasons we tend to think of gluconic as more of an all-round barrier restorer rather than an acid proper, even though it could be used as either!
As demonstrated above, acid type (chemistry) makes a lot of difference to how your formula will look, feel and perform. Pair that with the formula pH and your total concentration of acids and you have got all the information needed to make an effective acid functional formula.
Summing up pH, pH adjusting and formulating acid cosmetics
Most cosmetic formulations do not require the creator to have a deep knowledge of or appreciation for chemistry. This is because most cosmetic science is about creating environments for ingredients to co-exist together peacefully and without changing - avoiding rather than encouraging reactions and change. In this article however, we've focused on the times things do change, when chemical reactions do happen whether we planned them or not.
We've explored ways to maximise the efficacy of a formula that contains acids as its key actives, considering acid strength, concentration and make-up. We have also Investigated how and why the pH of a formula might change even when we feel we didn't add anything acidic or basic. We've looked at how pH adjustment works and what options we have for initiating that. But before all that we explored what causes pH, how it is measured and how little effect dilution has on this often talked about measure. Finally, and importantly, we've reminded ourselves of what pH means from a skin perspective. How we feel and experience pH and how our skin might respond to products whose pH is a little outside the ordinary.
While this has already been a very long article there is still so much more to tell you! That said, I'm confident that after reading this you should have a slightly better appreciation for the role of pH in your skincare and feel a bit more confident in knowing when and how to test pH and what that number means.
Chemistry is quite a difficult subject as it involves discussing things that you can't easily see and maybe can't even imagine. I've tried to make it easier to visualise with some analogies and real-life references but if you still don't get it don't worry too much as there's always someone out there who can help you with this aspect of formulating, product development and brand communication.
Amanda Foxon-Hill
